Physical Properties of Alcohols
Physical Properties of Alcohols: Overview
This Topic covers sub-topics such as Boiling Point of Alcohols, Water Solubility of Alcohols and, Physical Properties of Alcohols
Important Questions on Physical Properties of Alcohols
In allylic and benzylic alchohols, group is attached to
The image below shows the boiling point of first seven straight chain primary alcohols and first seven straight chain primary alkanes.
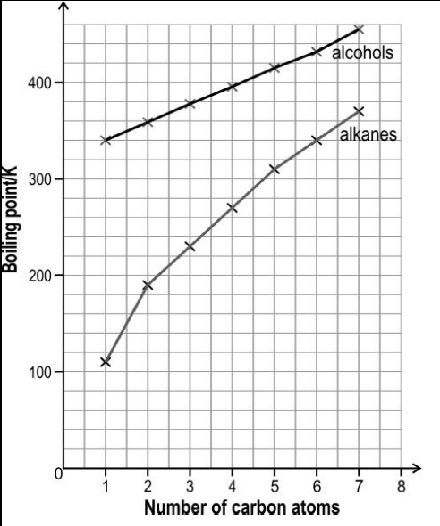
The boiling point of both the series increase monotonically with increasing size of the molecules. However, the slope of increment is different for both the series. Observe the above graph and answer the following question:
How will the boiling point graph for straight chain primary amines fare as compared to alcohols and alkanes?
The image below shows the boiling point of first seven straight chain primary alcohols and first seven straight chain primary alkanes.
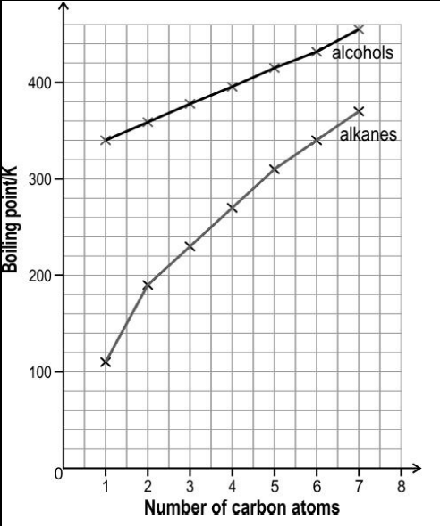
The boiling point of both the series increase monotonically with increasing size of the molecules. However, the slope of increment is different for both the series. Observe the above graph and answer the following question:
Will the graph look like almost the same if boiling point is replaced with melting point?
The image below shows the boiling point of first seven straight chain primary alcohols and first seven straight chain primary alkanes.
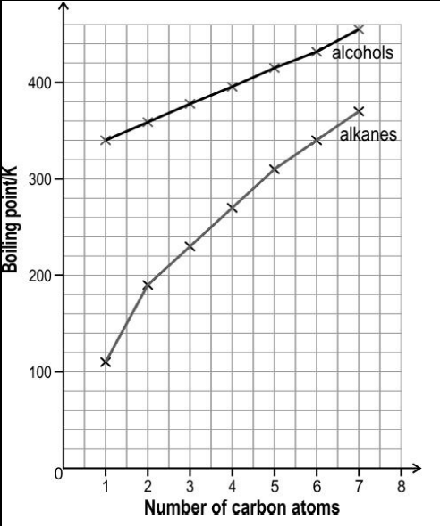
The boiling point of both the series increase monotonically with increasing size of the molecules. However, the slope of increment is different for both the series. Observe the above graph and answer the following question:
Can the two graphs ever intersect?
The image below shows the boiling point of first seven straight chain primary alcohols and first seven straight chain primary alkanes.
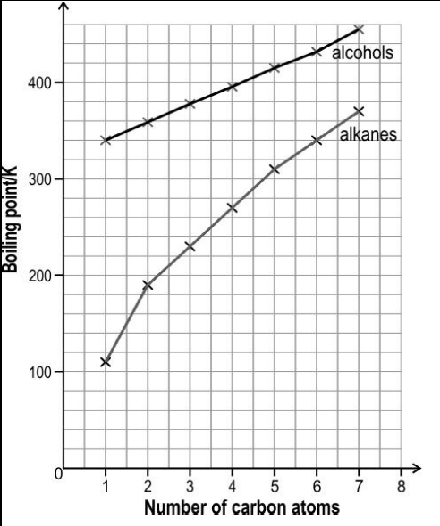
The boiling point of both the series increase monotonically with increasing size of the molecules. However, the slope of increment is different for both the series. Observe the above graph and answer the following question:
Why do the differences in boiling point between corresponding alcohols and alkanes get less as the number of carbon atoms increase?
The image below shows the boiling point of first seven straight chain primary alcohols and first seven straight chain primary alkanes.
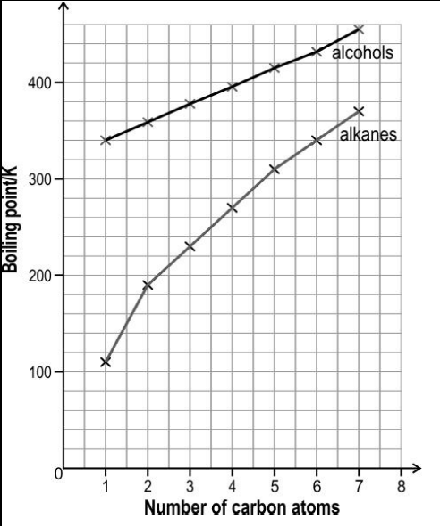
The boiling point of both the series increase monotonically with increasing size of the molecules. However, the slope of increment is different for both the series. Observe the above graph and answer the following question:
Why are the boiling point of alcohols higher than that of corresponding alkanes?
Lower alcohols are miscible with water because of the fact that _____
Assertion (A): An ether is more volatile than an alcohol of comparable molecular mass.
Reason (R): Ethers are polar in nature
Which is most VISCOUS ?
Arrange the following compounds in order of their increasing boiling points:
Alcohol which is most soluble in water is
Which of the following has highest boiling point?
The boiling points of alcohols decrease as the alkyl chain branching increases.
Suggest a reason for the large difference between boiling points of butanol and butanal, although they have almost the same solubility in water. Give reason.
Methanol is the most toxic alcohol.
Write any three physical properties of alcohols.
The % composition of ethanol in azeotropic mixture is _____.
Ethanol and water mixture is known as _____ azeotropes.
Mention the composition of ethanol azeotropes.
How does azeotropic mixture purified?
